CHEMICAL REACTIONS
EXOTHERMIC REACTIONS:
When one substance is brought together or mixed with another and the resulting interaction evolves or generates heat, the process is referred to as an exothermic reaction. An exothermic (exo- is a prefix meaning "out of") reaction is one where the energy flows out of the system into the environment. Combustion reactions are exothermic. Some exothermic reactions may require heating just to get started, and will then proceed on their own.
Exothermic reactions pose special hazards whether occurring in the open environment or within a closed container. In the open, the heat evolved will raise the temperature of the reactants, of any products of the reaction, and of surrounding materials. Since several properties of all substances are a function of temperature, such as pressure, the resulting higher temperatures may affect how the materials involved behave in the environment.
Heat will increase the vapor pressures of hazardous materials and the rate at which they vaporize. If very high temperatures are achieved, nearby combustible materials may ignite. Explosive materials, whether they are the reactants of the reaction or just nearby, may explode upon ignition or excessive heating.
Similar hazards are associated with exothermic reactions taking place in closed containers. In this case, however, increasing internal temperatures as well as the evolution of gases from the reaction may increase internal pressures to the point that the tank or container ruptures violently in an over-pressurization explosion, thus suddenly releasing large amounts of possibly flammable and/or toxic gases or vapors into the atmosphere. Such gases or vapors may also be released through ruptured pipes, opened relief valves or devices, or any other paths to the external environment.
REACTIONS WITH WATER OR AIR
Some of the most basic types of exothermic reactions occur when certain materials are dissolved in water.
Such substances have what is called a positive heat of solution. They do not transform to a different material, but simply generate heat while mixing. Some examples are sodium hydroxide (also called caustic soda) and sulfuric acid, which generates considerable heat to the point of causing some degree of "violence" when concentrated or pure materials are spilled into water. Other materials may ignite, evolve flammable gases, or otherwise react violently when in contact with water.
Knowledge of the reactivity of any substance with water is especially important when water is present in the spill area or a fire takes place and firefighters do not wish to make the situation worse by applying water to the flames or chemicals.
Several of the strong acids and related substances in this category of materials may evolve large amounts of fumes when in contact with water or moisture in the air. These fumes, which may consist of a mixture of fine droplets of acid in air and acid vapors, are usually highly irritating, corrosive, and heavier than air.
Many substances referred to as being pyrophoric will react violently or exothermically with air and are likely to ignite in a spontaneous fashion. Such substances (such as phosphorus) are commonly transported or stored in a manner that prevents exposure to air, often submerged in water or some type of compatible oil. Note: The fact that a substance can be safely stored under water in no way suggests that it may also be safely submerged in oil.
REACTIONS WITH COMBUSTIBLE ORGANIC MATERIALS:
Certain chemicals are known as strong oxidizing agents or oxidizers. They have the common characteristic of being able to decompose or oxidize organic materials and react with a variety of inorganic materials while generating heat, oxygen, flammable gases, and possibly toxic gases. If the heat generated is sufficient to ignite a combustible or flammable material, a fire or explosion may occur.
Another group of chemicals are referred to as strong reducing agents. These substances may evolve (produce, create) hydrogen upon reaction with many other chemicals, may evolve other flammable or toxic gases, and like oxidizing agents, may generate heat.
A fire or explosion may result if sufficient heat is generated to ignite a combustible or flammable substance.
Strong reducing agents and oxidizing agents should never be allowed to make contact without appropriate safeguards since they represent opposite extremes of chemical reactivity.
EXOTHERMIC POLYMERIZATION REACTIONS:
A few of the more common plastics in use on a widespread basis are polyethylene, polypropylene, polystyrene, and polyvinyl chloride (PVC). Although all are manufactured from liquids or gases, they are typically solids in their final form. They are respectively manufactured from ethylene, propylene, styrene, and vinyl chloride by means of a polymerization reaction in which molecules of these materials are linked together into long chains of molecules. As the chains become longer and begin connecting to each other, increasing the molecular weight of individual molecules, a solid plastic is formed.
Some chemicals capable of being polymerized have strong tendency to do so even under normal ambient conditions and are especially prone to polymerize if heated above a certain temperature or if contaminated by a catalyst or polymerization initiator, which in some cases might be a rather common substance such as water or rust.
Once polymerization starts, an exothermic chain reaction may occur that develops high temperatures and pressures within containers and which can lead to possible explosion or violent rupture of the container and/or discharge of flammable and/or toxic gases if safety and control systems malfunction are lacking.
The incident in Bhopal, India partially involved this type of reaction when a container of methyl isocyanate contaminated with water and chloroform began polymerizing. The reaction caused a large portion of isocyanate to vaporize into the air through a pressure relief system before it had a chance to polymerize.
Substances with the above tendency to self-polymerize or to undergo autocatalytic polymerization are transported or stored only while containing an amount of a substance called an inhibitor. Inhibitors act to inhibit, slow, or interfere with the chemical processes that can lead to a runaway uncontrolled polymerization reaction under normal conditions of transportation or storage. Inadvertent contamination or excessive heat, however, may overpower the inhibitor and allow the reaction to proceed.
EXOTHERMIC DECOMPOSITION REACTIONS:
While some chemical molecules can join together to form larger molecules via exothermic polymerization, others are unstable and can break apart in a runaway exothermic reaction once the process is initiated. Inhibitors may be used to slow the process down or to prevent its occurrence. Various contaminants or heat may overcome the inhibitors or otherwise start a uncontrolled reaction.
Decomposition and polymerization reactions are hazardous only if they somehow become uncontrolled and start a chain reaction that cannot be stopped with available equipment, materials, or safety systems. They are widely and safely conducted in chemical and other manufacturing plants across the nation on a daily bases without incident. It is only when control or safety systems break down or people make mistakes that problems develop.
CORROSIVE HAZARDS:
The process by which a chemical gradually erodes or dissolves another material is often referred to as corrosion. The word corrosive is also used descriptively to indicate that a substance may cause chemical burns of the skin, eyes, or other bodily tissues upon contact.
In evaluating whether one material is corrosive to another via reference to material safety data sheets, chemical company product bulletins, hazardous material data bases, or other reference sources, it is often important to place the time frame and rate of corrosion into the proper context.
ACIDS AND BASES (CORROSIVITY):
Corrosive materials are either acidic or basic. The relative degree of corrosivity is determined by the material's ability to dissociate or form ions in solution. Those that form the number of hydrogen ions [H+] are the strongest acids, while those that form hydroxide ions [OH-] are the strongest bases. The pH scale ranges from 0 to 14 and is logarithmic, meaning that each numerical increase on the pH scale represents a tenfold increase in acids and base concentration relative to pure water measured at 7.0 pH. A 6.0 pH is 10 times more acidic than 7.0 pH; a 5.0 is 100 times more acidic than 7.0 pH. The term "weak" when applied to acids and bases means that only a small portion of the substance in solution has formed ions. Dilute means that only a small amount of the substance is in solution and is not the same as "weak." A dilute solution of a strong acid may have a higher pH (less acidic) than a concentrated solution of a weak acid.
The following table lists pH values for some of the more common solutions:
|
SOLUTION
|
pH
|
|
Hydrochloric Acid(4%)
|
0
|
|
Gastric juices
|
1.6 to 1.8
|
|
Lemon juice
|
2.3
|
|
Vinegar
|
3
|
|
Coke
|
4
|
|
Milk
|
6.5
|
|
Water
|
7
|
|
Isopropyl Alcohol
|
7
|
|
Milk of magnesia
|
10.5
|
|
Sodium hydroxide (4%)
|
13
|
A buffer is a solution that resists changes in pH when either an acid or base is added. Buffer solutions are critical to the human organism. A pH imbalance in the blood can cause acidosis (low pH) which in extreme cases can cause shock, coma, and even death; or alkalosis (high pH) which in severe cases can lead to convulsions or death. A buffer solution contains two components in relatively large concentrations: a weak acid and a salt of that acid. Citric acid and sodium citrate form a buffer combination often found in commercial food products.
It is important to note that different acids can have different health effects on the human body.
For example: If splashed with a hydrochloric acid solution on 25 square inches of skin, an immediate
sensation of burning will take place and the tissue that was exposed will burn and decomposed until
the acid is flushed away or neutralized. While the exposed tissues will be damaged, there is no danger
of the exposure going "systemic" and killing the person. (Later, infection could be a problem though.)
Systemic means "body-wide," where the poison causes damage beyond the initial exposed area.
This could be something that contaminates the blood, or targets one or more vital organs.
If splashed with a 2% solution of Hydrofluoric Acid on 25 square inches of skin (5 inches by 5 inches),
the skin will not burn away like other acids, but the chemical will move to the bone to decompose the bone.
The effect will be systemic as the acid lowers the body's calcium level. Besides the extreme pain of the
bone being eaten away, without immediate medical attention, the victim will die. The lowering of calcium
levels in the blood by the Hydrofluoric Acid will also cause confusion, dizziness, and fainting.
|
IT IS EXTREMELY IMPORTANT FOR WORKERS TO KNOW AND UNDERSTAND THE ACUTE AND CHRONIC EFFECTS OF THE CHEMICALS THEY WILL BE LIKELY EXPOSED TO!
|
- Strong acids include hydrochloric acid (HCL), hydrofluoric acid (HF), nitric acid (HNO3), and sulfuric acid (H2SO4).
- Strong bases include sodium hydroxide (NaOH) and potassium hydroxide (KOH)
OTHER HAZARDOUS RESULTS OR PRODUCTS OF REACTIONS:
The combination of various chemicals may produce new chemicals with hazards quite different and possibly more hazardous than those associated with the original materials. Some combinations may result in spontaneous fires; spontaneous explosions; generation of toxic gases, liquids or solids; or generation of flammable gases, liquids or solids. It is necessary to look at hazardous materials on a fairly specific case-by-case basis to determine their reactivity hazards.
REACTIVITY:
The reactivity chart illustrated below is based on the National Fire Protection Association (NFPA)
704 System which now defines reactivity as instability while the Hazardous Materials
Identification System (HMIS) developed by the National Pain & Coatings Association still
defines reactivity as reactivity. [LINK http://www.nfpa.org/ ]
REACTIVITY/INSTABILITY HAZARD RATING CHART
|
Code No.
|
Hazard
|
Definition
|
|
1
|
Minimal
|
Normally stable even in fire conditions.
|
|
2
|
moderate
|
Normally unstable if heated; may react with water.
|
|
3
|
severe
|
Materials capable of detonation or explosion but require a strong initating source or which may react explosively with water.
|
|
4
|
extreme
|
Materials readily capable of explosive decomposition or detonation at normal temperature and pressure.
|
UNDERSTANDING THE BEHAVIOR OF SPILLED CHEMICALS
In order to understand how the impacts of chemical spills can be predicted,
some science and chemistry concepts must be examined.
VAPOR PRESSURE
Vapor pressure is the measurement of a particular liquid chemical's tendency
to vaporize. All liquids will evaporate, even thick liquids. Everyone is
familiar with the evaporation of liquids like water, gasoline, and the
solvents used in paints. When the paint dries, its solvents have all
evaporated away, leaving the dry finish. These individual chemicals all
evaporate at different rates, and they all have different vapor pressures.
And the vapor pressure for each increases as the temperature goes up. When
water is heated closer to its boiling point of 212 F, it evaporates at a
faster rate than water at room temperature. The vapor pressure for the water
increases as the water gets hotter. Those same paint solvents will dry
faster if the paint is warmer. It takes longer for paint to dry in a cold
house than a warm house.
Responders should beware of liquids with substantial (large) vapor
pressures, which is the primary measure of a chemical's tendency to
vaporize. Some chemicals evaporate faster than others.
Vapor pressure is also the measurement of the pressure exerted on the walls
of a container which is partially full of the liquid chemical and free of
any other vapor or gas. Higher temperatures cause increases in the vapor
pressure. Lower temperatures cause a decrease, and there is a direct
relationship between the temperature of any given substance and its vapor
pressure. The warmer a liquid is, the more it tends to evaporate.
Vapor pressure is most often expressed in units of millimeters of mercury
(mm Hg).
As a rule of thumb, the higher the vapor pressure, the further the distance
the vapor will disperse. There is a direct relationship between the vapor
pressure of an evaporating substance and the maximum concentration that its
vapor or gas may achieve when mixed with air in the open environment. In
other words, the concentration of the vapors of a spilled chemical will only
be so much in the air, and this concentration depends on the vapor pressure
of the chemical.
Higher vapor pressures above the surface of a substance require that more
molecules of the substance be physically present. Thus, if the vapor
pressure of the substance is known, the approximate maximum airborne
contaminant concentration it may attain can be calculated. Such
concentrations are most commonly expressed in units of percent in air by
volume, parts per million parts of air (ppm) by volume, or in milligrams of
chemical per cubic meter (mg/m3) of air.
 No matter what the vapor pressure of a spilled chemical, it can only
evaporate into the air if it is exposed to the air. So limiting the surface
of a spilled liquid is a common strategy in responding to a spill of a
liquid that is evaporating. Firefighters will dike (push dirt up to form a
pool) the area around a tank that is leaking liquid to limit the surface
area of the growing pool of liquid. The amount of surface area of a diked
pool of liquid is much less than an uncontained spill.
No matter what the vapor pressure of a spilled chemical, it can only
evaporate into the air if it is exposed to the air. So limiting the surface
of a spilled liquid is a common strategy in responding to a spill of a
liquid that is evaporating. Firefighters will dike (push dirt up to form a
pool) the area around a tank that is leaking liquid to limit the surface
area of the growing pool of liquid. The amount of surface area of a diked
pool of liquid is much less than an uncontained spill.
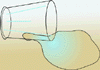 To illustrate this, look at a filled glass of water, and note the small
portion of the water that is in direct contact with the air. It is just a
fraction of the total amount of the water in the glass that is exposed. If
the glass of water is spilled, almost all of the water is exposed to the
air, and the water spreads out into a much larger pool. And obviously, it
would take longer for the water in the glass to evaporate than the spilled
water, because more of the water molecules are exposed to the air.
To illustrate this, look at a filled glass of water, and note the small
portion of the water that is in direct contact with the air. It is just a
fraction of the total amount of the water in the glass that is exposed. If
the glass of water is spilled, almost all of the water is exposed to the
air, and the water spreads out into a much larger pool. And obviously, it
would take longer for the water in the glass to evaporate than the spilled
water, because more of the water molecules are exposed to the air.
BOILING POINT
Any liquid will boil at the temperature at which its vapor pressure equals
the pressure being exerted by the environment onto the surface of the
liquid. This is why water boils at a lower temperature in high elevations.
So boiling points are related to the pressure exerted on the chemical.
Liquids in sealed containers (barrels, tanks) will remain as liquids when
heated above their normal boiling points although their vapor pressures may
become very dangerously high. If heating continues and the pressure is not
adequately relieved by a safety device, the pressure and temperature within
the tank may eventually rise to the point that some part or all of the
container may burst or rupture. Water will boil when heated to the boiling
point in an open pan on a stove, but if water is sealed inside a container
and heated to its boiling point, the water will cause the container to burst.
This is why firefighters will take steps to cool a tank near a fire--to
prevent a violent tank failure. When a tank bursts under this scenario, the
liquid tends to vaporize almost instantly, and if flammable, burn almost
instantly. One of the dangers associated with a tanker of propane is a
BLEVE, Boiling Liquid Expanding Vapor Explosion. A BLEVE is a catastrophic
explosion of a flammable fuel. (PICTURE)
If a spilled liquid is in an environment above its boiling point, it will
rapidly boil and expand, sometimes explosively. An example of this is
Ammonia. Ammonia boils at -28.17 degrees Fahrenheit. If Ammonia spills,
there may be a second phase reaction, which can cause a cloud of Ammonia to
expand and move very quickly. Ammonia is lighter than air, and once it warms
up, it tends to move up and way into the atmosphere.
Often, the available data about a chemical will indicate its boiling point,
but, just like water, a chemical in liquid form will evaporate at
temperatures far below the boiling point.
ATMOSPHERIC PRESSURE
The weight of the air is on everything, and the atmospheric pressure at sea
level is more than at the top of a mountain. The weight of the air at sea
level has been measured. The atmospheric pressure at sea level is 14.7
pounds per square inch, expressed as psi. This means that for every square
inch of surface an object has, there are 14.7 pounds of pressure pushing down.
This sea level pressure is also expressed as one atmosphere. In the United
States, the most common relative scale of measurement is in terms of gauge
pressure, where a reading of zero matches an absolute pressure of one
standard atmosphere. In this system, an absolute pressure of 15.7 psi would
be expressed as 1.0 pound per square inch - gauge, or 1.0 psig for short.
[15.7 - 14.7 = 1.0] The pressure inside a tank of chemical will be usually
expressed in these terms.
Any gas or vapor entering the atmosphere will quickly adjust its volume to
achieve a total pressure of the atmosphere it is released in. The vapors
from a spill of a chemical in a higher elevation will travel further than
one at sea level, all other factors being the same.
A tank of a liquefied gas, when opened to the atmosphere, will spew out many
times the original volume of the tank. Unless the tank is sealed, the
liquefied gas will escape as a gas until the pressure of the atmosphere
outside the tank is the same as inside the tank. A tank of propane, when
emptied directly into the air, will still contain propane, but the pressure
of the the remaining propane will be at the same atmospheric pressure as the
outside air.
 To illustrate how gases behave when they escape from opened containers, open
a bottle of a carbonated beverage. When one is
opened, bubbles of carbon dioxide are seen forming and rising to the
surface. Before opening the bottle, the carbon dioxide was dissolved into
the liquid under pressure. When the bottle is opened, the pressure is
released, and the carbon dioxide is seen escaping. If a person shook the
bottle before opening, the carbon dioxide will escape more rapidly, spraying
the liquid quite a distance. There is no way to look at the bottle of
carbonated beverage and tell if it has been shook up or not. When modeling
what a plume from a tank of chemicals might be, these same uncertainties are
present.
To illustrate how gases behave when they escape from opened containers, open
a bottle of a carbonated beverage. When one is
opened, bubbles of carbon dioxide are seen forming and rising to the
surface. Before opening the bottle, the carbon dioxide was dissolved into
the liquid under pressure. When the bottle is opened, the pressure is
released, and the carbon dioxide is seen escaping. If a person shook the
bottle before opening, the carbon dioxide will escape more rapidly, spraying
the liquid quite a distance. There is no way to look at the bottle of
carbonated beverage and tell if it has been shook up or not. When modeling
what a plume from a tank of chemicals might be, these same uncertainties are
present.
When a tank of liquids vents gases into the outside atmosphere, the tank of
liquids itself is cooled. This lowers the vapor pressure of the liquid in
the tank, and may significantly slow the release of the gases from the tank.
It also creates a special hazard because the leak may be more difficult to
detect.
VAPOR DENSITY
Chemicals have different molecular formulas and makeup. Vapor density is the
ratio of the density of a pure gas or vapor to the density of air. Vapors or
gases with a vapor density less than air tend to float above the air, moving
up and away from the spilled chemical and the ground. This is just like oil
floating on water because oil is less dense than water. Vapors or gases with
a vapor density higher than air tend to sink and move along the ground. When
the vapor density is close to that of air, the behavior can be more
unpredictable, but is treated as if the gas is heavier than air.
MOLECULAR WEIGHT
The molecular weight of a chemical depends on what elements make up its
chemical formula. The molecular weight will vastly influence the behavior of
a spilled chemical, including vapor pressure. The heavier the molecule of a
chemical that is evaporating, the slower it will be to evaporate and float
away. The heavier the molecules, the more likely they will be to move along
the ground rather than upwards.
SPECIAL CHEMICAL REACTIONS SOFTWARE
When working at a facility, a facility making chemical process changes, or
even a facility that is merely rearranging chemicals stored at the facility,
the facility workers should be aware of, and consider the compatibility of,
the various chemicals that may be stored next to each other at the facility.
This is important because the inadvertent mixing of incompatible chemicals
can cause fires, explosions, poison gases to form, and other unexpected
outcomes. It is important to consider what possible mixing of chemicals
might occur in the event of a spill, fire, or hazardous materials incident.
To help understand potential reactions where more than one chemical may be
involved in a spill scenario, the Chemical Reactivity Worksheet has been
developed. It can be found on the web at:
http://response.restoration.noaa.gov/chemaids.html, or at
http://response.restoration.noaa.gov/chemaids/react.html. This can be
downloaded from the Internet. It includes a database of reactivity
information for more than 4,000 common hazardous chemicals. The database
includes information about the special hazards of each chemical and about
whether a chemical reacts with air, water, or other materials. It also
includes a way to virtually "mix" chemicals to find out what dangers could
arise from accidental mixing.
 No matter what the vapor pressure of a spilled chemical, it can only
evaporate into the air if it is exposed to the air. So limiting the surface
of a spilled liquid is a common strategy in responding to a spill of a
liquid that is evaporating. Firefighters will dike (push dirt up to form a
pool) the area around a tank that is leaking liquid to limit the surface
area of the growing pool of liquid. The amount of surface area of a diked
pool of liquid is much less than an uncontained spill.
No matter what the vapor pressure of a spilled chemical, it can only
evaporate into the air if it is exposed to the air. So limiting the surface
of a spilled liquid is a common strategy in responding to a spill of a
liquid that is evaporating. Firefighters will dike (push dirt up to form a
pool) the area around a tank that is leaking liquid to limit the surface
area of the growing pool of liquid. The amount of surface area of a diked
pool of liquid is much less than an uncontained spill.
 To illustrate this, look at a filled glass of water, and note the small
portion of the water that is in direct contact with the air. It is just a
fraction of the total amount of the water in the glass that is exposed. If
the glass of water is spilled, almost all of the water is exposed to the
air, and the water spreads out into a much larger pool. And obviously, it
would take longer for the water in the glass to evaporate than the spilled
water, because more of the water molecules are exposed to the air.
To illustrate this, look at a filled glass of water, and note the small
portion of the water that is in direct contact with the air. It is just a
fraction of the total amount of the water in the glass that is exposed. If
the glass of water is spilled, almost all of the water is exposed to the
air, and the water spreads out into a much larger pool. And obviously, it
would take longer for the water in the glass to evaporate than the spilled
water, because more of the water molecules are exposed to the air.
 To illustrate how gases behave when they escape from opened containers, open
a bottle of a carbonated beverage. When one is
opened, bubbles of carbon dioxide are seen forming and rising to the
surface. Before opening the bottle, the carbon dioxide was dissolved into
the liquid under pressure. When the bottle is opened, the pressure is
released, and the carbon dioxide is seen escaping. If a person shook the
bottle before opening, the carbon dioxide will escape more rapidly, spraying
the liquid quite a distance. There is no way to look at the bottle of
carbonated beverage and tell if it has been shook up or not. When modeling
what a plume from a tank of chemicals might be, these same uncertainties are
present.
To illustrate how gases behave when they escape from opened containers, open
a bottle of a carbonated beverage. When one is
opened, bubbles of carbon dioxide are seen forming and rising to the
surface. Before opening the bottle, the carbon dioxide was dissolved into
the liquid under pressure. When the bottle is opened, the pressure is
released, and the carbon dioxide is seen escaping. If a person shook the
bottle before opening, the carbon dioxide will escape more rapidly, spraying
the liquid quite a distance. There is no way to look at the bottle of
carbonated beverage and tell if it has been shook up or not. When modeling
what a plume from a tank of chemicals might be, these same uncertainties are
present.