UNDERSTANDING THE BEHAVIOR OF SPILLED CHEMICALS
In order to understand how the impacts of chemical spills can be predicted,
some science and chemistry concepts must be examined.
VAPOR PRESSURE
Vapor pressure is the measurement of a particular liquid chemical's tendency
to vaporize. All liquids will evaporate, even thick liquids. Everyone is
familiar with the evaporation of liquids like water, gasoline, and the
solvents used in paints. When the paint dries, its solvents have all
evaporated away, leaving the dry finish. These individual chemicals all
evaporate at different rates, and they all have different vapor pressures.
And the vapor pressure for each increases as the temperature goes up. When
water is heated closer to its boiling point of 212 F, it evaporates at a
faster rate than water at room temperature. The vapor pressure for the water
increases as the water gets hotter. Those same paint solvents will dry
faster if the paint is warmer. It takes longer for paint to dry in a cold
house than a warm house.
Responders should beware of liquids with substantial (large) vapor
pressures, which is the primary measure of a chemical's tendency to
vaporize. Some chemicals evaporate faster than others.
Vapor pressure is also the measurement of the pressure exerted on the walls
of a container which is partially full of the liquid chemical and free of
any other vapor or gas. Higher temperatures cause increases in the vapor
pressure. Lower temperatures cause a decrease, and there is a direct
relationship between the temperature of any given substance and its vapor
pressure. The warmer a liquid is, the more it tends to evaporate.
Vapor pressure is most often expressed in units of millimeters of mercury
(mm Hg).
As a rule of thumb, the higher the vapor pressure, the further the distance
the vapor will disperse. There is a direct relationship between the vapor
pressure of an evaporating substance and the maximum concentration that its
vapor or gas may achieve when mixed with air in the open environment. In
other words, the concentration of the vapors of a spilled chemical will only
be so much in the air, and this concentration depends on the vapor pressure
of the chemical.
Higher vapor pressures above the surface of a substance require that more
molecules of the substance be physically present. Thus, if the vapor
pressure of the substance is known, the approximate maximum airborne
contaminant concentration it may attain can be calculated. Such
concentrations are most commonly expressed in units of percent in air by
volume, parts per million parts of air (ppm) by volume, or in milligrams of
chemical per cubic meter (mg/m3) of air.
 No matter what the vapor pressure of a spilled chemical, it can only
evaporate into the air if it is exposed to the air. So limiting the surface
of a spilled liquid is a common strategy in responding to a spill of a
liquid that is evaporating. Firefighters will dike (push dirt up to form a
pool) the area around a tank that is leaking liquid to limit the surface
area of the growing pool of liquid. The amount of surface area of a diked
pool of liquid is much less than an uncontained spill.
No matter what the vapor pressure of a spilled chemical, it can only
evaporate into the air if it is exposed to the air. So limiting the surface
of a spilled liquid is a common strategy in responding to a spill of a
liquid that is evaporating. Firefighters will dike (push dirt up to form a
pool) the area around a tank that is leaking liquid to limit the surface
area of the growing pool of liquid. The amount of surface area of a diked
pool of liquid is much less than an uncontained spill.
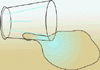 To illustrate this, look at a filled glass of water, and note the small
portion of the water that is in direct contact with the air. It is just a
fraction of the total amount of the water in the glass that is exposed. If
the glass of water is spilled, almost all of the water is exposed to the
air, and the water spreads out into a much larger pool. And obviously, it
would take longer for the water in the glass to evaporate than the spilled
water, because more of the water molecules are exposed to the air.
To illustrate this, look at a filled glass of water, and note the small
portion of the water that is in direct contact with the air. It is just a
fraction of the total amount of the water in the glass that is exposed. If
the glass of water is spilled, almost all of the water is exposed to the
air, and the water spreads out into a much larger pool. And obviously, it
would take longer for the water in the glass to evaporate than the spilled
water, because more of the water molecules are exposed to the air.
BOILING POINT
Any liquid will boil at the temperature at which its vapor pressure equals
the pressure being exerted by the environment onto the surface of the
liquid. This is why water boils at a lower temperature in high elevations.
So boiling points are related to the pressure exerted on the chemical.
Liquids in sealed containers (barrels, tanks) will remain as liquids when
heated above their normal boiling points although their vapor pressures may
become very dangerously high. If heating continues and the pressure is not
adequately relieved by a safety device, the pressure and temperature within
the tank may eventually rise to the point that some part or all of the
container may burst or rupture. Water will boil when heated to the boiling
point in an open pan on a stove, but if water is sealed inside a container
and heated to its boiling point, the water will cause the container to burst.
This is why firefighters will take steps to cool a tank near a fire--to
prevent a violent tank failure. When a tank bursts under this scenario, the
liquid tends to vaporize almost instantly, and if flammable, burn almost
instantly. One of the dangers associated with a tanker of propane is a
BLEVE, Boiling Liquid Expanding Vapor Explosion. A BLEVE is a catastrophic
explosion of a flammable fuel.
If a spilled liquid is in an environment above its boiling point, it will
rapidly boil and expand, sometimes explosively. An example of this is
Ammonia. Ammonia boils at -28.17 degrees Fahrenheit. If Ammonia spills,
there may be a second phase reaction, which can cause a cloud of Ammonia to
expand and move very quickly. Ammonia is lighter than air, and once it warms
up, it tends to move up and way into the atmosphere.
Often, the available data about a chemical will indicate its boiling point,
but, just like water, a chemical in liquid form will evaporate at
temperatures far below the boiling point.
ATMOSPHERIC PRESSURE
The weight of the air is on everything, and the atmospheric pressure at sea
level is more than at the top of a mountain. The weight of the air at sea
level has been measured. The atmospheric pressure at sea level is 14.7
pounds per square inch, expressed as psi. This means that for every square
inch of surface an object has, there are 14.7 pounds of pressure pushing down.
This sea level pressure is also expressed as one atmosphere. In the United
States, the most common relative scale of measurement is in terms of gauge
pressure, where a reading of zero matches an absolute pressure of one
standard atmosphere. In this system, an absolute pressure of 15.7 psi would
be expressed as 1.0 pound per square inch - gauge, or 1.0 psig for short.
[15.7 - 14.7 = 1.0] The pressure inside a tank of chemical will be usually
expressed in these terms.
Any gas or vapor entering the atmosphere will quickly adjust its volume to
achieve a total pressure of the atmosphere it is released in. The vapors
from a spill of a chemical in a higher elevation will travel further than
one at sea level, all other factors being the same.
A tank of a liquefied gas, when opened to the atmosphere, will spew out many
times the original volume of the tank. Unless the tank is sealed, the
liquefied gas will escape as a gas until the pressure of the atmosphere
outside the tank is the same as inside the tank. A tank of propane, when
emptied directly into the air, will still contain propane, but the pressure
of the the remaining propane will be at the same atmospheric pressure as the
outside air.
 To illustrate how gases behave when they escape from opened containers, open
a bottle of a carbonated beverage. When one is
opened, bubbles of carbon dioxide are seen forming and rising to the
surface. Before opening the bottle, the carbon dioxide was dissolved into
the liquid under pressure. When the bottle is opened, the pressure is
released, and the carbon dioxide is seen escaping. If a person shook the
bottle before opening, the carbon dioxide will escape more rapidly, spraying
the liquid quite a distance. There is no way to look at the bottle of
carbonated beverage and tell if it has been shook up or not. When modeling
what a plume from a tank of chemicals might be, these same uncertainties are
present.
To illustrate how gases behave when they escape from opened containers, open
a bottle of a carbonated beverage. When one is
opened, bubbles of carbon dioxide are seen forming and rising to the
surface. Before opening the bottle, the carbon dioxide was dissolved into
the liquid under pressure. When the bottle is opened, the pressure is
released, and the carbon dioxide is seen escaping. If a person shook the
bottle before opening, the carbon dioxide will escape more rapidly, spraying
the liquid quite a distance. There is no way to look at the bottle of
carbonated beverage and tell if it has been shook up or not. When modeling
what a plume from a tank of chemicals might be, these same uncertainties are
present.
When a tank of liquids vents gases into the outside atmosphere, the tank of
liquids itself is cooled. This lowers the vapor pressure of the liquid in
the tank, and may significantly slow the release of the gases from the tank.
It also creates a special hazard because the leak may be more difficult to
detect.
VAPOR DENSITY
Chemicals have different molecular formulas and makeup. Vapor density is the
ratio of the density of a pure gas or vapor to the density of air. Vapors or
gases with a vapor density less than air tend to float above the air, moving
up and away from the spilled chemical and the ground. This is just like oil
floating on water because oil is less dense than water. Vapors or gases with
a vapor density higher than air tend to sink and move along the ground. When
the vapor density is close to that of air, the behavior can be more
unpredictable, but is treated as if the gas is heavier than air.
MOLECULAR WEIGHT
The molecular weight of a chemical depends on what elements make up its
chemical formula. The molecular weight will vastly influence the behavior of
a spilled chemical, including vapor pressure. The heavier the molecule of a
chemical that is evaporating, the slower it will be to evaporate and float
away. The heavier the molecules, the more likely they will be to move along
the ground rather than upwards.
 No matter what the vapor pressure of a spilled chemical, it can only
evaporate into the air if it is exposed to the air. So limiting the surface
of a spilled liquid is a common strategy in responding to a spill of a
liquid that is evaporating. Firefighters will dike (push dirt up to form a
pool) the area around a tank that is leaking liquid to limit the surface
area of the growing pool of liquid. The amount of surface area of a diked
pool of liquid is much less than an uncontained spill.
No matter what the vapor pressure of a spilled chemical, it can only
evaporate into the air if it is exposed to the air. So limiting the surface
of a spilled liquid is a common strategy in responding to a spill of a
liquid that is evaporating. Firefighters will dike (push dirt up to form a
pool) the area around a tank that is leaking liquid to limit the surface
area of the growing pool of liquid. The amount of surface area of a diked
pool of liquid is much less than an uncontained spill.
 To illustrate this, look at a filled glass of water, and note the small
portion of the water that is in direct contact with the air. It is just a
fraction of the total amount of the water in the glass that is exposed. If
the glass of water is spilled, almost all of the water is exposed to the
air, and the water spreads out into a much larger pool. And obviously, it
would take longer for the water in the glass to evaporate than the spilled
water, because more of the water molecules are exposed to the air.
To illustrate this, look at a filled glass of water, and note the small
portion of the water that is in direct contact with the air. It is just a
fraction of the total amount of the water in the glass that is exposed. If
the glass of water is spilled, almost all of the water is exposed to the
air, and the water spreads out into a much larger pool. And obviously, it
would take longer for the water in the glass to evaporate than the spilled
water, because more of the water molecules are exposed to the air.
 To illustrate how gases behave when they escape from opened containers, open
a bottle of a carbonated beverage. When one is
opened, bubbles of carbon dioxide are seen forming and rising to the
surface. Before opening the bottle, the carbon dioxide was dissolved into
the liquid under pressure. When the bottle is opened, the pressure is
released, and the carbon dioxide is seen escaping. If a person shook the
bottle before opening, the carbon dioxide will escape more rapidly, spraying
the liquid quite a distance. There is no way to look at the bottle of
carbonated beverage and tell if it has been shook up or not. When modeling
what a plume from a tank of chemicals might be, these same uncertainties are
present.
To illustrate how gases behave when they escape from opened containers, open
a bottle of a carbonated beverage. When one is
opened, bubbles of carbon dioxide are seen forming and rising to the
surface. Before opening the bottle, the carbon dioxide was dissolved into
the liquid under pressure. When the bottle is opened, the pressure is
released, and the carbon dioxide is seen escaping. If a person shook the
bottle before opening, the carbon dioxide will escape more rapidly, spraying
the liquid quite a distance. There is no way to look at the bottle of
carbonated beverage and tell if it has been shook up or not. When modeling
what a plume from a tank of chemicals might be, these same uncertainties are
present.